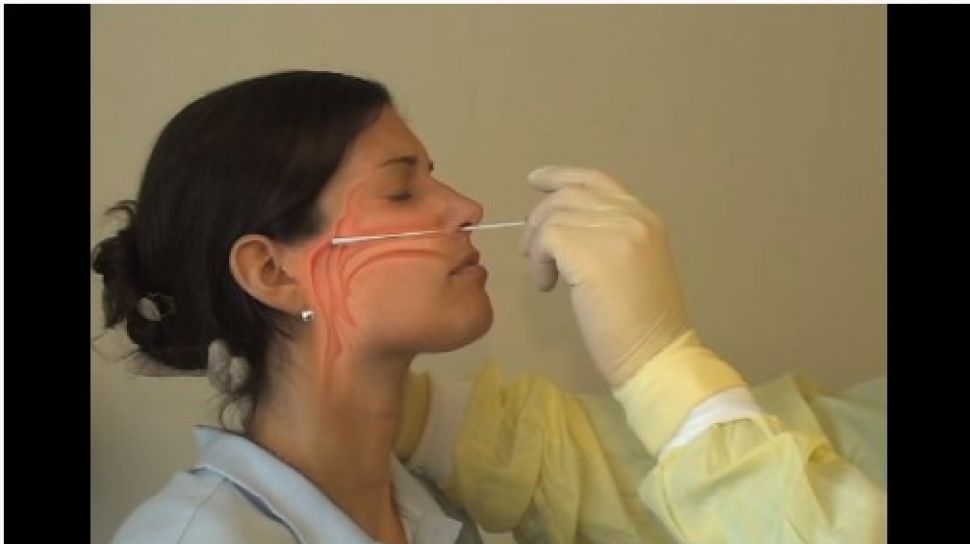
Covid 19 Test Machine For Sale - Covid-19 Realtime Info
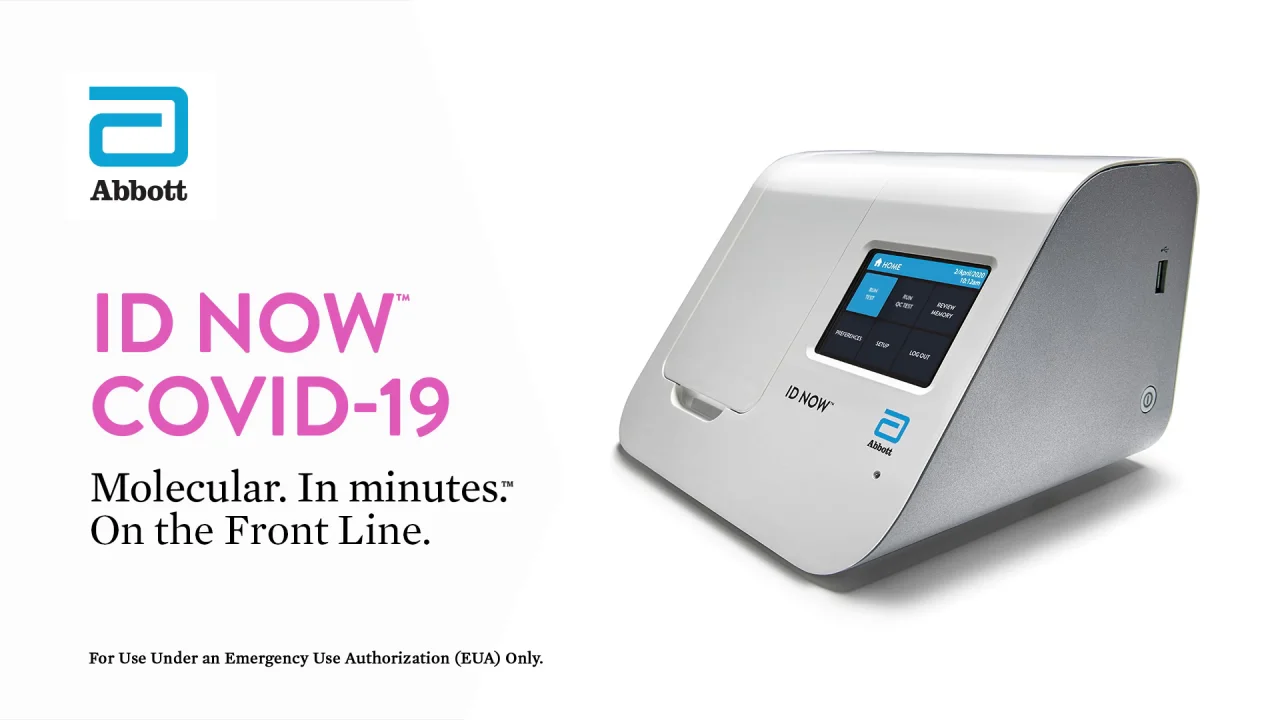
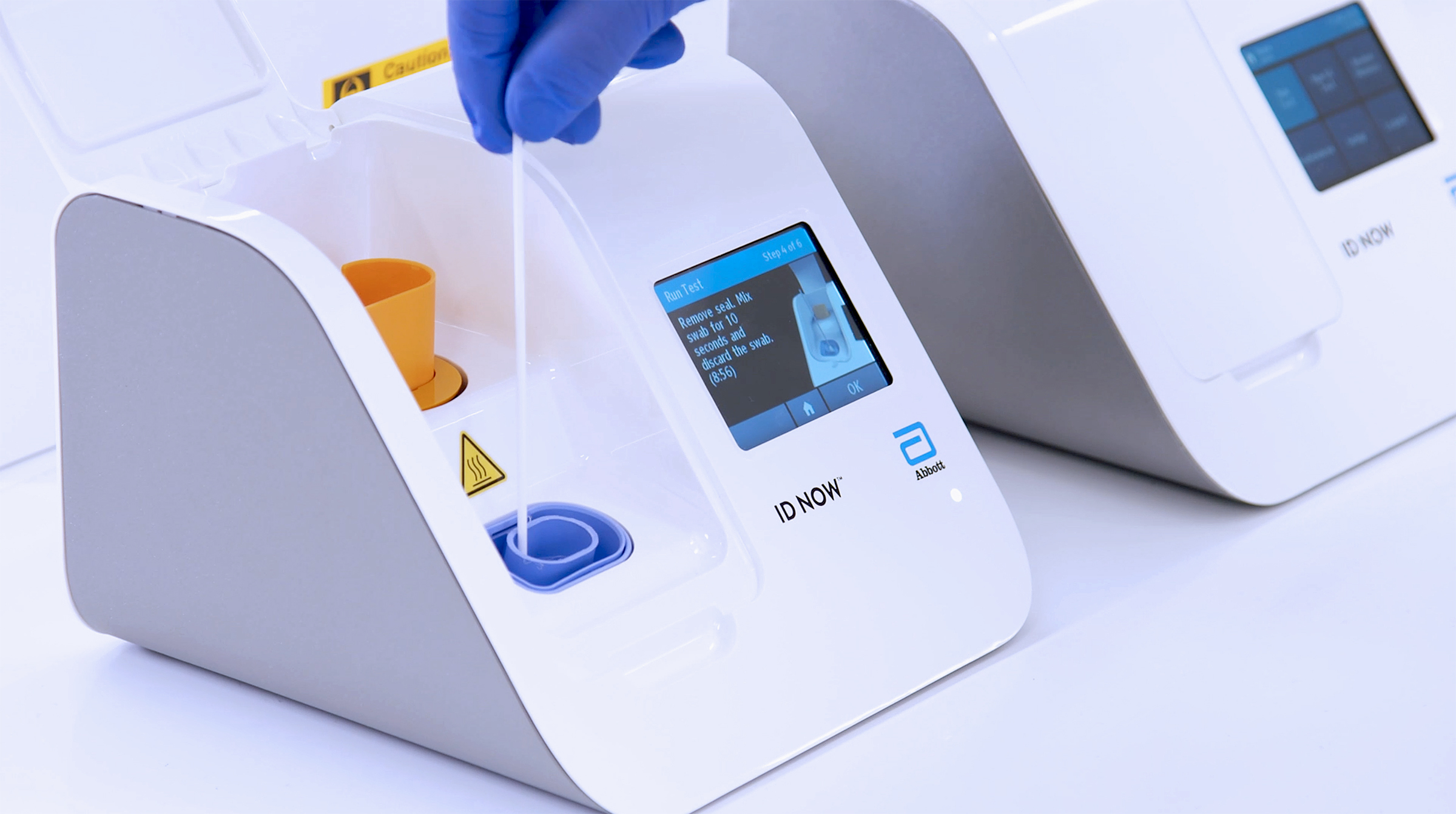
Headquartered in minneapolis mn qiagen supplies the qiastat dx respiratory sars cov 2 panel test which can test 22 different diseases including covid 19.

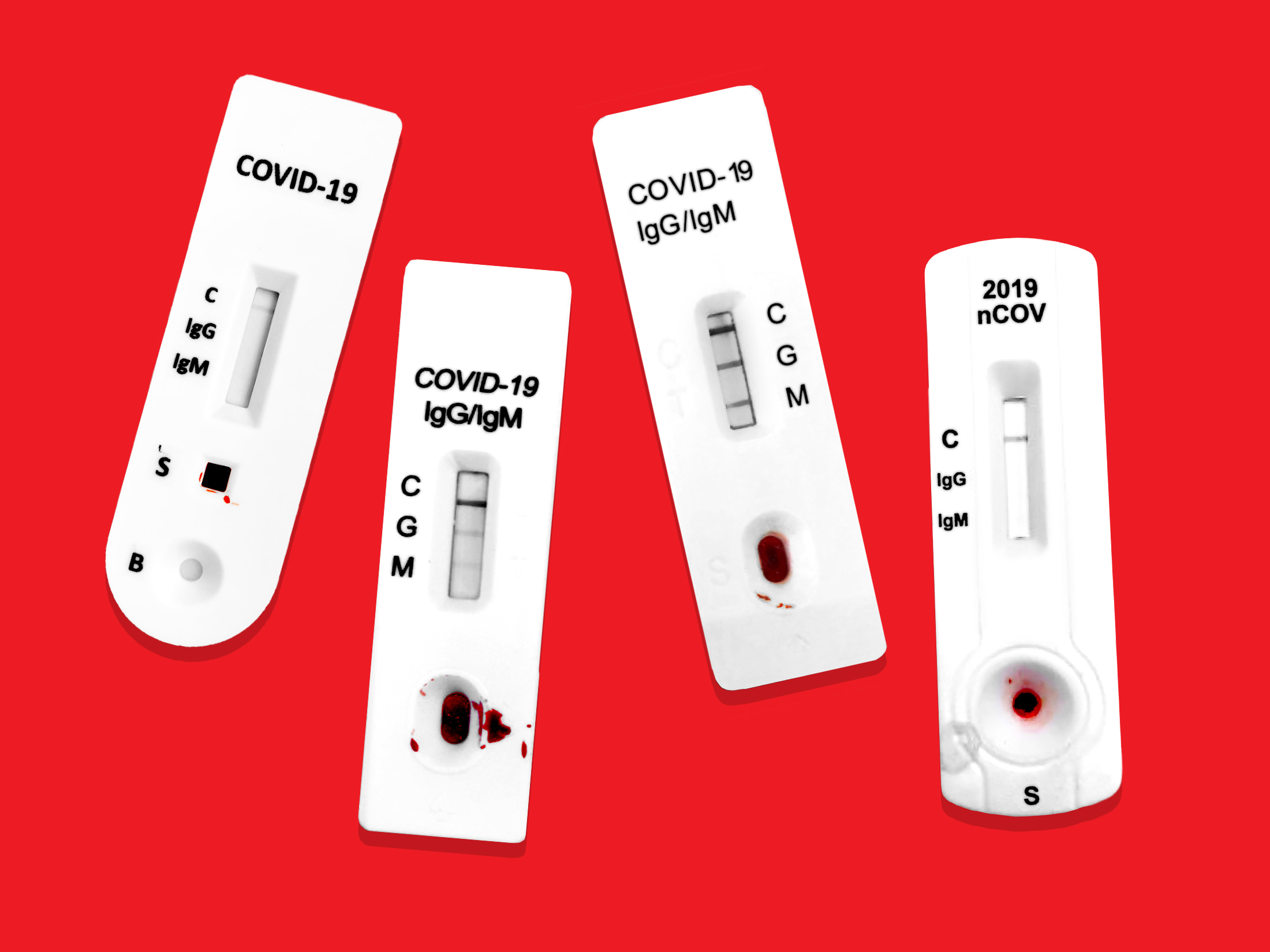
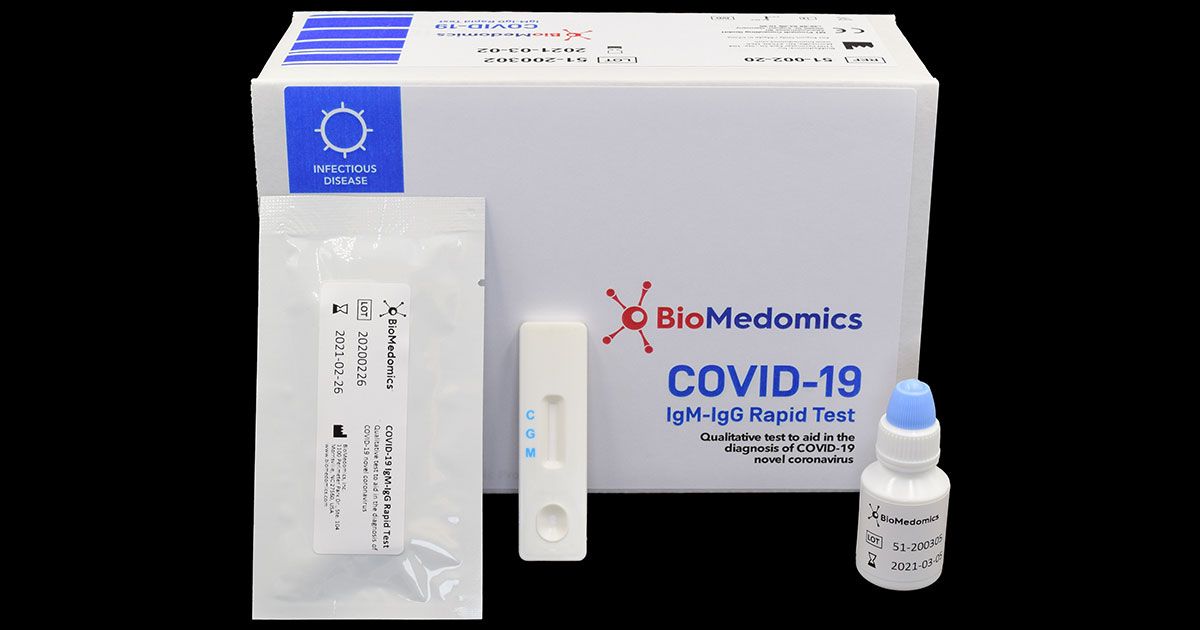
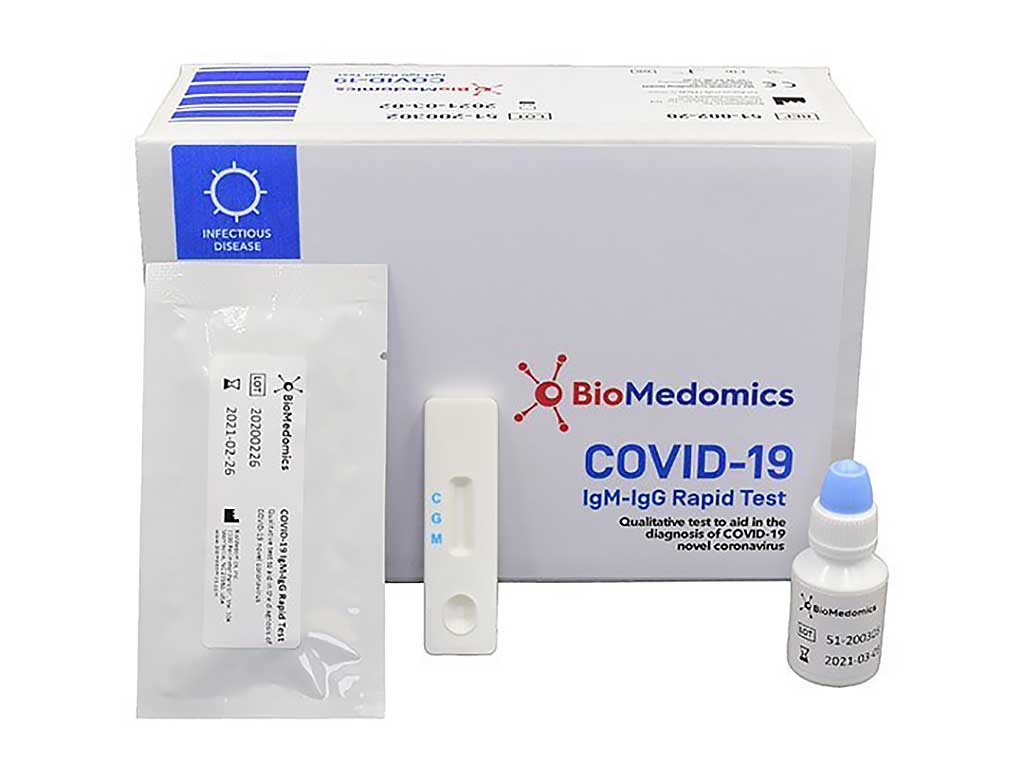
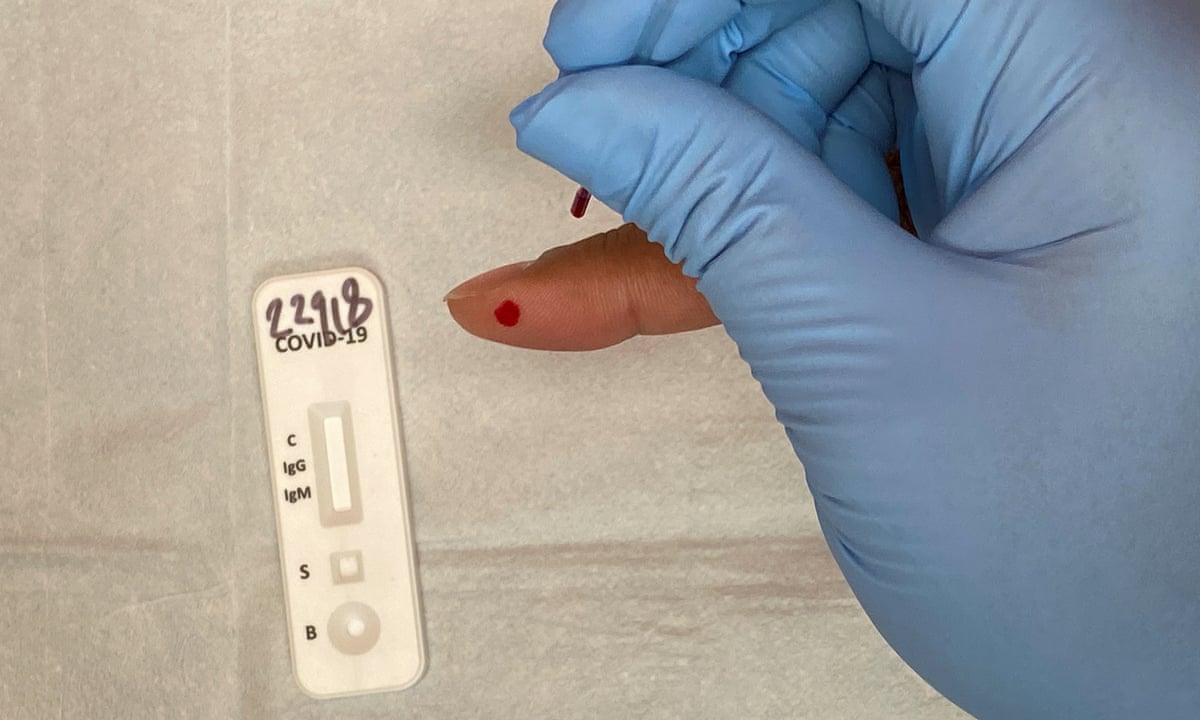
Covid 19 test machine for sale. Resources for covid 19 test kits last updated march 19 currently there is still a shortage of covid 19 test kits in the united states. The test was validated against a panel of previously frozen samples consisting of twenty six 26 sars cov 2 antibodies both igm and igg positive and eighty 80 antibody negative plasma samples. The onsite covid 19 iggigm rapid test has 971 sensitivity. This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection andor diagnosis of covid 19 under section 564b 1 of the act 21 usc.
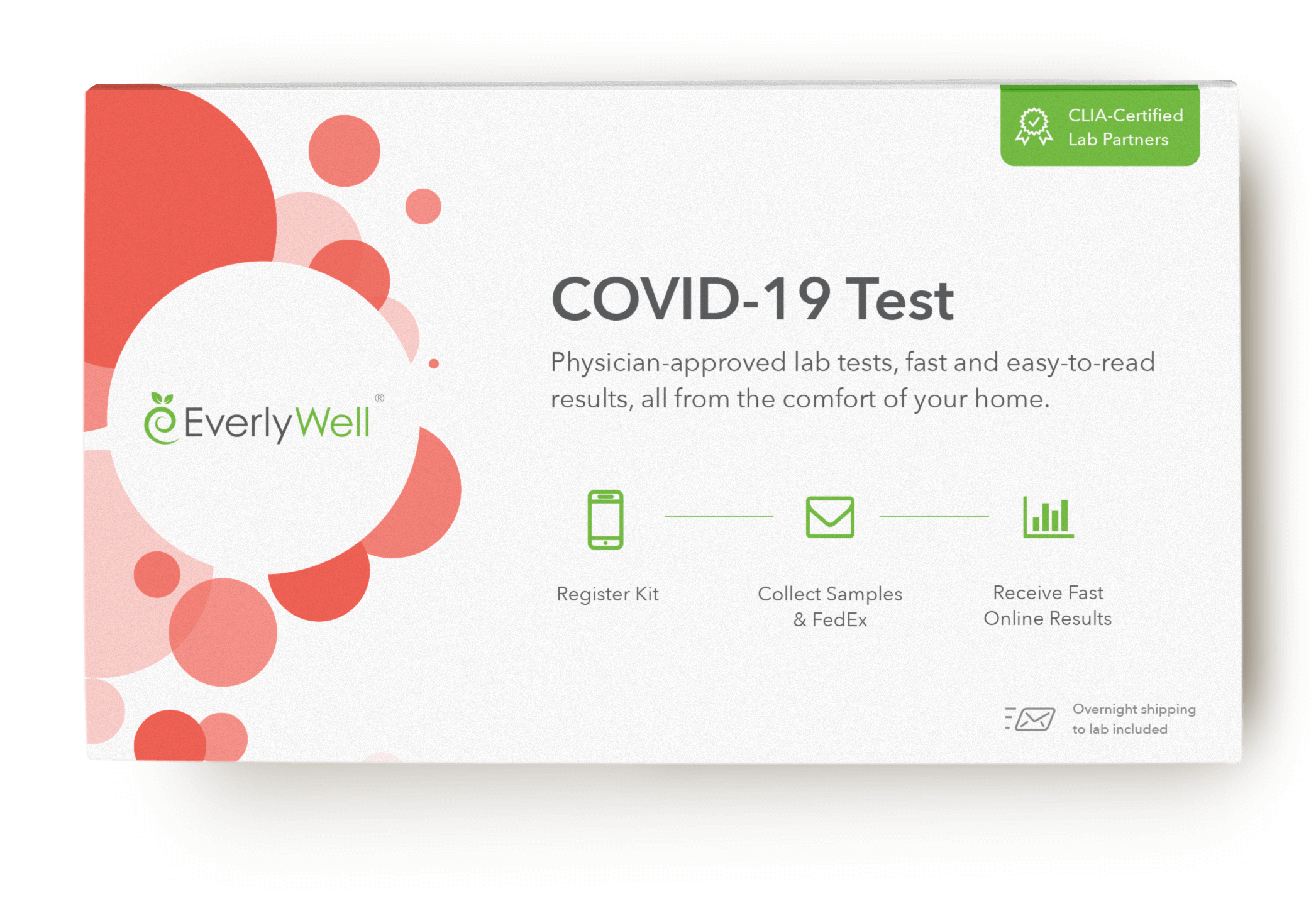
Together with our partner randox we have succeeded in developing this innovative rapid test within a very short time frame and we are now in a position to offer it to the market. This test has been authorized only for the presence of igm and igg antibodies against sars cov 2 not for any other viruses or pathogens. The partner labs processing the covid 19 test are complying with the fdas emergency use authorization guidance for covid 19 testing. Learn more about our at home coronavirus test kit today.
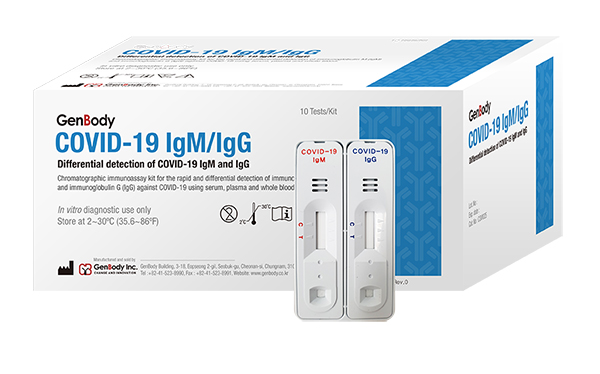
Buy pcr based covid 19 rapid test kits. The onsite covid 19 iggigm rapid test is designed for initial screening by detecting anti sars cov 2 igg and igm antibodies in either human serum plasma or whole blood within 15 minutes. In addition to this test cdc has developed a diagnostic test that can be used to detect sars cov 2 influenza a and influenza b viruses at the same time. The covid 19 igm igg rapid test is intended to test igm and igg separately.
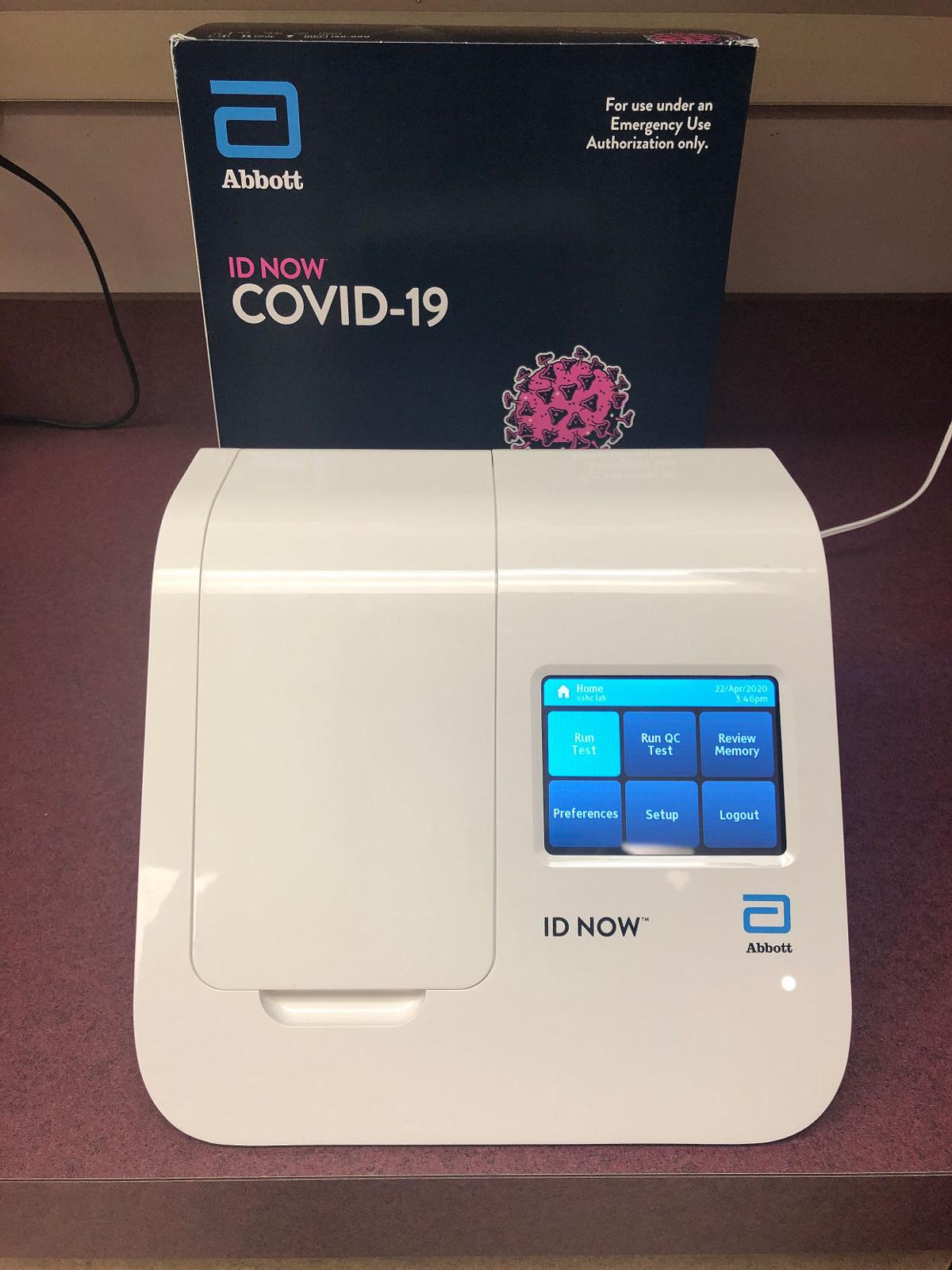
This test is called the cdc influenza sars cov 2 flu sc2 multiplex assay. The test is run on the companys qiastat dx analyzer. Boschs rapid covid 19 test is the result of collaboration between the companys bosch healthcare solutions subsidiary and the northern irish medical technology company randox laboratories ltd. Onsite covid 19 iggigm rapid test.
Test for the coronavirus that causes covid 19 from the convenience and safety of your own home. They will be used by healthcare workers at point of care in order to rapidly diagnose patients directing those who test positive for the infection. The test has been authorized only for the detection of nucleic acid from sars cov 2 not for any other viruses or pathogens and is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostic tests for detection andor diagnosis of covid 19 under section 564b. The samba ii machines developed by diagnostics for the real world provide a simple and accurate system for the diagnosis of infection with sars cov 2 the virus that causes the disease covid 19.














































/cdn.vox-cdn.com/uploads/chorus_asset/file/19856029/IDNOW_INACTION3_macro_300dpi_1200x628.jpg)













/data/photo/2020/03/08/5e64ec24a38ee.jpg)